Conformity assessment
as a requirement for the market placement of a medical device
Unlike medicinal products, medical devices are not approved by an authority in the European Economic Area (EEA).
Medical devices do not require an official authorisation. This distinguishes them from medicinal products. Medicinal products can only be placed on the market after an official authorisation by the authorities.
In order for a manufacturer to be allowed to place his medical device on the market in the EEA, he must submit a declaration of conformity for the product. In this declaration, the manufacturer confirms that the product complies with the applicable legal requirements, in particular with regard to safety as well as technical and medical performance. If this is the case, the manufacturer may affix the CE mark to the product.

Before the declaration of conformity is drawn up, the product undergoes a conformity assessment procedure. A conformity assessment procedure can consist of one or more modules, which the manufacturer can select depending on the risk characteristics of the product. The modules concern the product's technical documentation, the manufacturer's quality management system, individual type examination, production quality assurance or product conformity to a type examination. The aim is to demonstrate that the manufacturer has suitable processes in place to ensure the safety and performance of its products on a permanent basis - and that the individual product is safe and performs effectively.
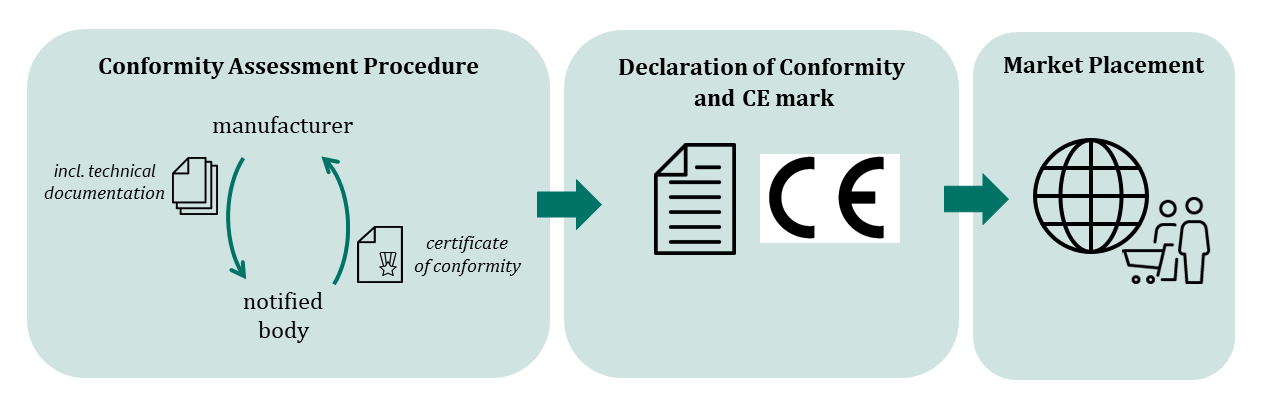
For many products, a notified body must be involved in the conformity assessment procedure. Notified bodies examine and evaluate the conformity assessment to be carried out by the manufacturer and certify conformity with the legal requirements according to uniform assessment standards (certificate of conformity).
In Germany, the Central Authority of the Laender for Health Protection with regard to Medicinal Products and Medical Devices (Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten, ZLG) is responsible for the designation and monitoring of notified bodies.
Further information on notified bodies as well as overviews of the notified bodies in Germany and the EEA:
NANDO (New Approach Notified and Designated Organisations) home page
Which conformity assessment procedure is applicable for a specific product and whether a notified body has to be involved depends primarily on the risk classification of the product. Furthermore, a distinction must be made between medical devices, which are subject to the European Regulation for Medical Devices, and in vitro diagnostics, which are subject to the European Regulation for In Vitro Diagnostics.
Medical devices
For each medical device, the manufacturer must determine the risk of health damage resulting from the use of his product and the severity of this potential damage. Legal requirements then determine whether the product is to be assigned to a low risk class or a high risk class. For medical devices, there are risk classes I, IIa, IIb and III. Risk class I corresponds to the lowest risk, class III to the highest risk. In risk class I, a further distinction is made between "with measuring function" (class Im) and "sterile" (class Is) as well as "reusable surgical instruments".
The assignment to a risk class is made by the manufacturer himself according to legally defined rules. The starting point for determining the risk and thus assigning the product to a risk class is always the product’s intended purpose. This takes into account, for example, whether a product is invasive, how long it is used or whether it emits energy.
See Annex VIII of Regulation (EU) 2017/745 for the complete classification rules for medical devices. Laws and ordinances
Further information on the subject of classification:
It depends on the risk class of the product which module or module combinations of the conformity assessment procedure may be applied. For devices of the lowest risk class (class I), which have neither a measuring function nor the suffix "sterile" and which are not reusable surgical instruments, the manufacturer may carry out the conformity assessment procedure without the involvement of a notified body. For all other risk classes, the involvement of a notified body is mandatory.
See Article 52 of Regulation (EU) 2017/745 for the complete rules on the application of the conformity assessment procedures. Laws and ordinances
The BfArM is not involved in the conformity assessment procedure. More information on notified bodies as well as overviews on the notified bodies in Germany and the EEA that are designated under the Medical Devices Regulation:
Notified Bodies for medical devices (MDR)
Clinical investigations
As part of the conformity assessment procedure, the safety and performance of the product must also be demonstrated under clinical conditions of use. The evidence can be provided by clinical data derived from literature, from clinical experience or from already completed clinical investigations. If these data are not sufficient, a clinical investigation is conducted on volunteer subjects.
A prerequisite for conducting a clinical investigation is a favourable opinion of the responsible ethics committee. In addition, depending on the device and study and the suitable legal procedure, either an approval by the competent higher federal authority (BfArM) or only a notification to this institution is necessary.
More information on clinical investigations:
Responsible ethics committees for clinical investigations:
In vitro diagnostics
In vitro diagnostics directive
According to the law in force up to and including May 25, 2022 (Directive 98/79/EG, In Vitro Diagnostics Directive, IVDD) IVDs were not divided into classes. According to Art. 9 IVDD, the decisive factor for the choice of the conformity assessment procedure module was whether the product was intended for self-testing or whether it was listed in Annex II of the IVDD (so-called high-risk IVD). Only for these products, a notified body had to be involved.
IVDs placed on the market under Directive 98/79/EC may remain on the market for a transition period. The length of the transition period depends on which risk class the IVD would fall into according to the IVDR(see below). As a rule of thumb, the higher the expected risk class, the shorter the transition period.
In vitro diagnostics regulation
Since Regulation (EU) 2017/746 (In Vitro Diagnostics Regulation, IVDR), IVDs have been divided into risk classes. There are the risk classes A to D. Additional regulations apply to specific IVDs: For IVDs intended for use by lay persons (product for self-testing), for near-patient tests, and for companion diagnostics.
For each IVD, the manufacturer must determine the risk, whether the use of his product may cause health damage and how severe this damage may be. The assignment to a risk class is made by the manufacturer of the IVD himself according to legally defined rules. The starting point for the risk determination and thus the assignment of the product to a risk class is always the intended purpose of the product. This takes into account, for example, whether the IVD is used to detect a transmissible, potentially life-threatening pathogen, whether it is a product for blood grouping or tissue typing, or whether it is a product for the early detection of certain types of cancer.
See Annex VIII of Regulation (EU) 2017/746 for the complete classification rules. Laws and ordinances
Further information on the subject of classification:
The risk class of the IVD determines which module or module combinations of the conformity assessment procedure can be applied. For IVDs of the lowest risk class (class A), which do not bear the suffix "sterile", the manufacturer can carry out the conformity assessment procedure without the involvement of a notified body. For all other risk classes, the involvement of a notified body is mandatory.
See Article 48 of Regulation (EU) 2017/746 for the complete rules on the application of the conformity assessment procedure for IVDs. Laws and ordinances
The BfArM is not involved in the conformity assessment. More information on notified bodies as well as overviews of the notified bodies in Germany and the EEA designated under the Regulation for In Vitro Diagnostic Medical Devices:
Notified Bodies for in vitro diagnostic medical devices (IVDR)
Performance studies
As part of the conformity assessment procedure, the safety and performance of the product must be clinically demonstrated. Evidence can be provided by clinical data derived from literature, clinical experience or already completed performance studies. If these data are not sufficient, a performance study is conducted on sample material provided by volunteer subjects.
A prerequisite to conduct a performance study is a favourable opinion of the responsible ethics committee. In addition, depending on the type of device and study and the suitable legal procedure, either an approval by the competent higher federal authority (BfArM or PEI) or only a notification to this institution is necessary.
Further information on performance studies:
Responsible ethics committees for performance studies:
Special approvals
Under strict conditions, a manufacturer may, in exceptional cases, place a medical device on the market without a conformity assessment procedure via a so-called special approval. This procedure is only considered if health protection cannot be guaranteed in any other way. This was the case, for example, during the coronavirus pandemic (mouth-nose protection, rapid antigen tests for self-testing).
