Interesting facts
What is a Digital Health Application (DiGA)?
Digital health applications (DiGA – in German: “Digitale Gesundheitsanwendungen”) open up a wide range of possibilities, both regarding the diagnosis and treatment of diseases as well as supporting a self-determined, healthy lifestyle. Thus, we can consider DiGA to be "digital assistants" in the hands of the patient.
A DiGA is a CE-marked medical device that has the following properties:
- Medical device of the risk class I or IIa (according to the Medical Device Regulation (MDR) or the transitional regulation Medical Device Directive (MDD)). Information as to "when is an App a medical device?" can be found at Differentiation and Classification.
- The main function of the DiGA is based on digital technologies.
- The medical purpose is mainly achieved by way of its digital function.
- The DiGA supports the recognition, monitoring, treatment or alleviation of diseases or the recognition, treatment, alleviation or compensation of injuries or disabilities.
- The DiGA is used by the patient alone or by patient and healthcare provider together.
These requirements are defined in Section 33a of the German Social Code Book V (Fünftes Buch Sozialgesetzbuch, SGB V).
Legal Basis: Digital Healthcare Act (DVG) and Digital Health Applications Ordinance (DiGAV)
The coming into force of the Digital Healthcare Act (Digitale-Versorgung-Gesetz, DVG) on 19 December 2019 marked the introduction into the healthcare system of the "app on prescription" for patients (Sections 33a and 139e of the German Social Code Book V). This means that approximately 73 million persons covered by the German statutory health insurance are entitled to use a DiGA prescribed by a physician or psychotherapist and are reimbursed by the health insurance.
Prerequisite for the above is that a DiGA must have successfully completed the assessment of the BfArM leading to a listing in a directory of reimbursable digital health applications (DiGA directory). The Federal Ministry of Health (Bundesministerium für Gesundheit, BMG) has regulated the details of this procedure in the supplementary legal regulation, the Digital Health Applications Ordinance (Digitale Gesundheitsanwendungen-Verordnung, DiGAV). (Please note that only the German version of the Digital Health Applications Ordinance is avaliable.)
(Please note that only the German version is available.)
BfArM Assessment Procedure
The procedure is designed as a fast-track process: Within a three-month period at the most, starting with the filing of the complete application, the BfArM has to assess the DiGA. The essence of this assessment is the examination of the manufacturer’s statements about the product qualities – from data protection to interoperability and user friendliness - and the examination of the evidence of the positive healthcare effect of the DiGA provided by the manufacturer. These are effects through which the state of a patient's health or his/her possibilities for dealing with his/her disease are improved by the use of the DiGA.

The BfArM has prepared a guide with important information on process sequence, application procedure and the provision of evidence.
Please note that the current version of the guide is only available in German. The current information of data processing outside Germany will be updated in the guide with the next version. Please note the handout of data processing outside Germany.
Data processing outside Germany
In the following, the BfArM presents its current legal position with regard to the processing of personal data outside Germany in connection with the use of digital health applications (DiGA).
We would like to point out that further coordination on this topic is currently taking place on a European level and with the data protection authorities, among others, and that this may have an impact on the legal assessment.
Possible deviating specifications, e.g. of data protection authorities, may require technical adjustments of the DiGA with a corresponding obligation to notify the BfArM in order to ensure continued compliance with the specifications on data protection and to avoid a possible removal of the DiGA from the directory according to §139e SGB V.
Support and advice by BfArM
The BfArM offers advice on the prerequisites for listing in the DiGA directory in order to ensure comprehensive support for applicants and to provide early assistance in the compilation of significant documents and data for listing in the directory. In a kick-off meeting, questions applicants have regarding individual products that go beyond the explanations given in the guide can be discussed. In addition, the BfArM gives advice regarding procedural issues as well as concerning the notification of significant changes to a DiGA.
For planning reasons, advice meetings on fast-track procedures will initially take place Wednesday and Thursday afternoons (except for public holidays) from 1 to 4 pm. Please take these time slots into account and indicate your preferred dates when applying for a kick-off meeting regarding the fast-track procedure. Requests are processed in the order in which they are received. Please send the filled out form stating the dates you prefer to: innovation@bfarm.de
The BfArM's Guide on the Fast-Track Process: A Transparent Overview of the Procedure and Help in Interpreting the Requirements
In its guide, the BfArM provides a summary of the regulations that can be found in various passages of the SGB V, the DiGAV and the Annexes to the DiGAV. It also sets out how it will regularly interpret the normative specifications of DVG and DiGAV, thus creating transparency regarding the specific requirements to be fulfilled in the procedure. This guide serves as a reliable basis for action, both for applicants and for the BfArM. At the same time, the guide is also designed in such a way as to allow all interested parties a comprehensive overview of the basis of assessment and consequently of the (quality) characteristics of a DiGA. We will continuously amend, extend and further develop the guide on the basis of the experience gained.
Should you have any kind of feedback to the guide or suggestions for additional or more precise information please address these to leitfaden@bfarm.de or diga@bfarm.de .
(Please note that the English text of the guide as well as the English translation of the respective legal texts are provided by the BfArM as a service and that only the German text version is binding.)
Please note that the current version of the guide is only available in German. The current information of data processing outside Germany will be updated in the guide with the next version. Please note the handout of data processing outside Germany.
The application procedure and the electronic application portal
Here you can access the BfArM application portal for the fast-track procedure for DiGA pursuant to Section 139e SGB V. Please bear in mind that all applications and notifications can only be submitted electronically through this portal; submission via e-mail or postal services is not possible.
It is the aim of the electronic portal to help you with the management of your applications and notifications to the BfArM and to provide you with the best possible support in submitting them.
Additionally, we are providing you with a guide for filling out the form containing further information and assistance regarding the use of the application portal. Specifically, you will find recommendations on preparations to be made prior to submitting the application,
- an overview of the information and documents to be submitted within the electronic application procedure for listing DiGA in the directory pursuant to Section 139e SGB V and
- specification of the contents of individual fields of the electronic submission process.
- This is intended to support manufacturers of DiGA in the efficient submission of complete and formally correct applications.
Gradually, feedback on the guide for filling out the form, which in its current version focusses mainly on the registration in the portal and the application for preliminary or final listing, will be amended by addition of instructions on how to complete the form for an application for extension of trial phase, notification of significant changes or deletion from the DiGA directory.
In this context we appreciate your feedback regarding the user-friendliness of the portal as well as references to possible problems and suggestions for improvement (also pertaining to the guide for filling out the form), under e-mail address diga@bfarm.de .
The DiGA Directory
This directory presents important information for DiGA users as well as for physicians and psychotherapists concerning those DiGA that have met all the requirements and have successfully completed the BfArM's assessment procedure. This creates a high degree of transparency, which allows well-informed decisions and enables confidence that the system is trustworthy.
The information in the directory includes - aside from general information such as the name of the product and the manufacturer - statements on positive healthcare effects, i.e. as to how the state of a patient's health or his/her possibilities for dealing with his/her disease improve by using the DiGA, as well as information on prescription and reimbursement of the DiGA.
Information on the Provision of Prescription-relevant Data
The essential identifying feature of a DiGA for prescription is the pharmaceutical central number (Pharmazentralnummer, PZN), which is assigned to the DiGA by the BfArM on the basis of different prescription units:
Prescription units of DiGA
One DiGA can include different prescription units, similar, e.g. to dosages and package sizes of medicinal products, that the physician or psychotherapist can prescribe for a patient. Each prescription unit carries its own ID and can thus be prescribed specifically. Individual prescription units may vary depending on the features of the DiGA, differing e.g. with regard to the following characteristics:
- Does the prescription include hardware (e.g. heart rate monitor, ECG sensor technology)?
- Is it a starter package with a one-time additional effort (e.g. special data collection once at the beginning or one-time installation of special hardware) or the continuation of an application that has already been started (e.g. application for further 30 days)?
- For which period of time (e.g. 30, 60, 90 days) is the DiGA prescribed?
- In cases in which a DiGA includes several modules: which module is supposed to be prescribed (e.g. "Joint fit - Module Knee", "Joint-fit - Module Shoulder")?
DiGA-ID and DiGA-Prescription-Unit-ID in the Directory
In accordance with Section 20 sub-section 1 of the DiGAV, the BfArM assigns a unique directory number to each DiGA included in the directory pursuant to Section 139e SGB V. This number identifies the DiGA within the directory, thus referencing it to the respective directory entry.
Accordingly, each DiGA (e.g. the DiGA "Joint-fit") is assigned a 5-digit numerical DiGA-ID (e.g. 12345) upon listing in the directory.
In order to allow unambiguous identification of different prescription units of one and the same DiGA in the directory, each prescription unit is assigned an 8-digit numerical DiGA-Prescription-Unit-ID (DiGA-VE-ID) consisting of the 5-digit DiGA-ID directly followed by a consecutive 3-digit prescription unit number (e.g. "Joint-fit - Knee Module - 30-day prescription": 12345001, "Joint-fit - Knee Module - 60-day prescription": 12345002, "Joint-fit - Shoulder Module - 30-day prescription": 12345003).
The DiGA-VE-ID thus shows that it is a specific prescription unit (last three digits) of a specific DiGA listed in the directory (first five digits).
Pharmaceutical Central Number (PZN)
In addition to the prescription of DiGA and their specific prescription units by physicians or psychotherapists, each DiGA prescription unit is assigned a unique 8-digit numerical Pharmaceutical Central Number (PZN) upon inclusion in the directory. This number represents the established standard for the identification of e.g. different dosages and package sizes of medicinal products in Germany and has therefore already been implemented in all practice management systems (PVS).
This PZN is assigned centrally by the Information Agency for Drug Specialities - IFA GmbH (http://www.ifaffm.de/), which provides the BfArM with the required PZN for allocation to the DiGA prescription units to be listed in the directory pursuant to Section 139e SGB V additionally to the DiGA-VE-ID.
Regardless of how the data is transferred to the practice management systems, the PZN represents the ID relevant for the prescription of DiGA. Thus DiGA or their individual prescription units can be prescribed using the PZN listed in the directory pursuant to Section 139e SGB V and subsequently displayed in the PVS by printing or writing them manually on the corresponding prescription form (sample 16).
Procedure for the Provision of Prescription-relevant Data by the BfArM
In accordance with Section 20 sub-section 1 DiGAV, the BfArM lists in its directory pursuant to Section 139e SGB V the DiGA that are reimbursable in the statutory health insurance system in accordance with Section 33a sub-section 1 SGB V. This listing in the DiGA directory includes both the DiGA-ID and the DiGA-VE-ID as well as the PZN. The BfArM is already working intensively on the development of a corresponding interface for the direct electronic provision of prescription-relevant data for the PVS. Until this interface is available and has also been implemented in the software of the PVS manufacturers, the established data delivery channel via the IFA will be used. Accordingly, when a new entry is made or an entry in directory V is changed, the BfArM immediately delivers a corresponding data set to the IFA, which then makes it available for use in PVS, so that they are available there for selection and prescription to physicians and psychotherapists prescribing DiGA.
In order to achieve the quickest possible provision and to avoid corresponding programming effort on the part of IFA and PVS manufacturers, only data fields used for medicinal products and medical aids are used as they are already implemented in the PVS. You will find an overview of the data fields transmitted from the BfArM to the IFA including an explanatory example in this Table. Since the PZN is provided directly to the BfArM by the IFA and not to the manufacturer of the respective DiGA, the BfArM is listed as "provider" (“Anbieter”) in the IFA system.
As soon as the direct interface is available, BfArM will announce this and the switch of the data transfer procedures will be made in coordination with the participating institutions and associations. Unaffected by this, the PZN will remain the identifying feature for prescription.
Facts and Figures of the DiGA directory
Here you will find weekly updated facts and figures regarding the DiGA directory and the BfArM's DiGA fast-track procedure. You can find information on how many applications for provisional or final listing in the DiGA directory the BfArM has received and how many of the assessments have already been completed, e.g. by listing in the DiGA directory.
For reasons of data protection we cannot give any details on the current applications, e.g. which manufacturers have submitted applications or what DiGA they refer to, but you will find all information on those DiGA that have successfully completed the BfArM's assessment procedure in the DiGA directory.
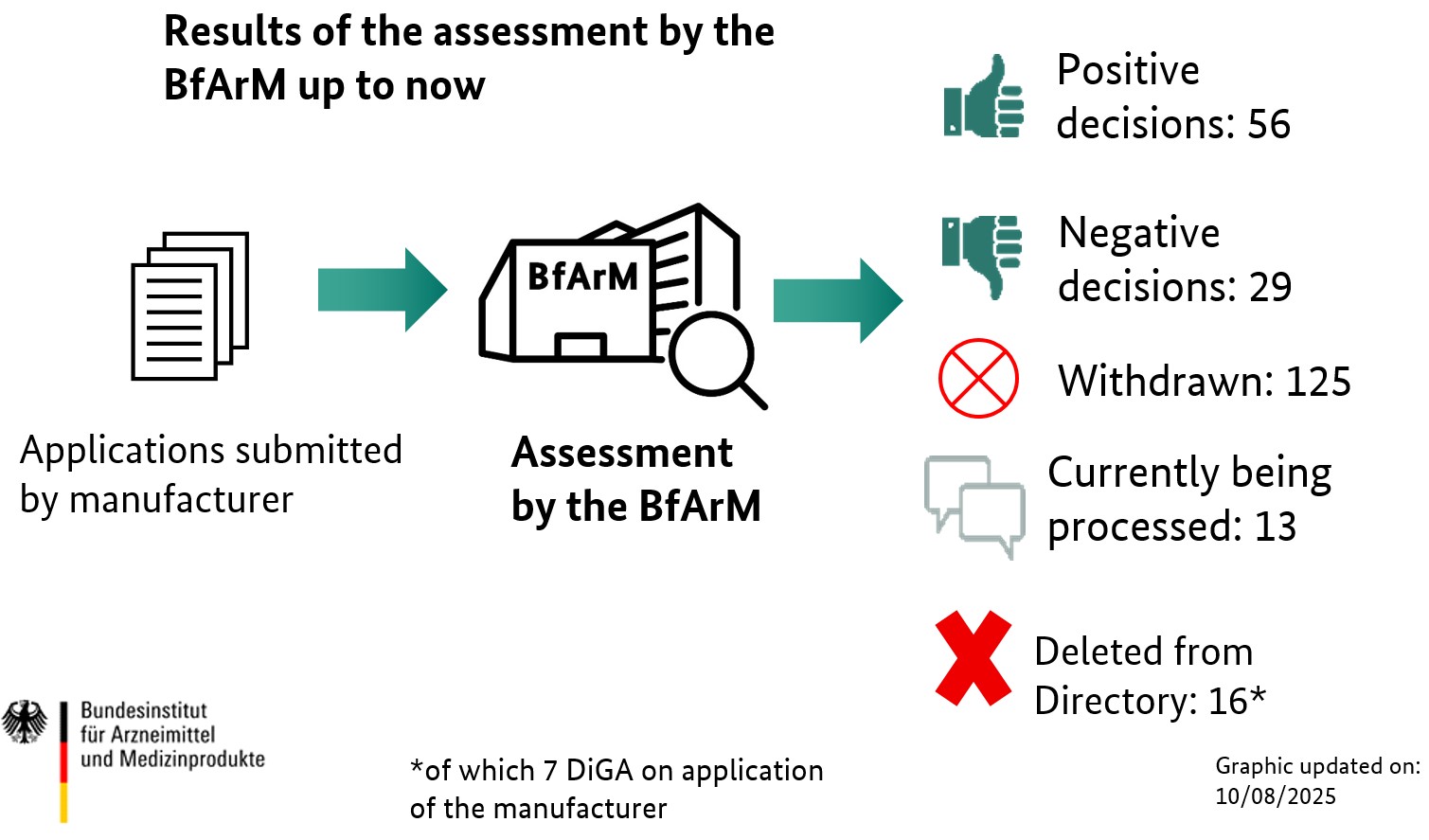
This chart shows how many applications for listing in the DiGA directory have been submitted to the BfArM since the beginning of the procedure on 27 May 2020. You can also see how many of these applications were submitted directly for final listing in the directory and how many were submitted for provisional listing during a trial phase for final generation of evidence. The Manufacturer or applicant takes the decision as to which type of application he submits to the BfArM. Further information and more in-depth explanations can be found here on our website as well as in our guide.
Please note that the current version of the guide is only available in German. The current information of data processing outside Germany will be updated in the guide with the next version. Please note the handout of data processing outside Germany.
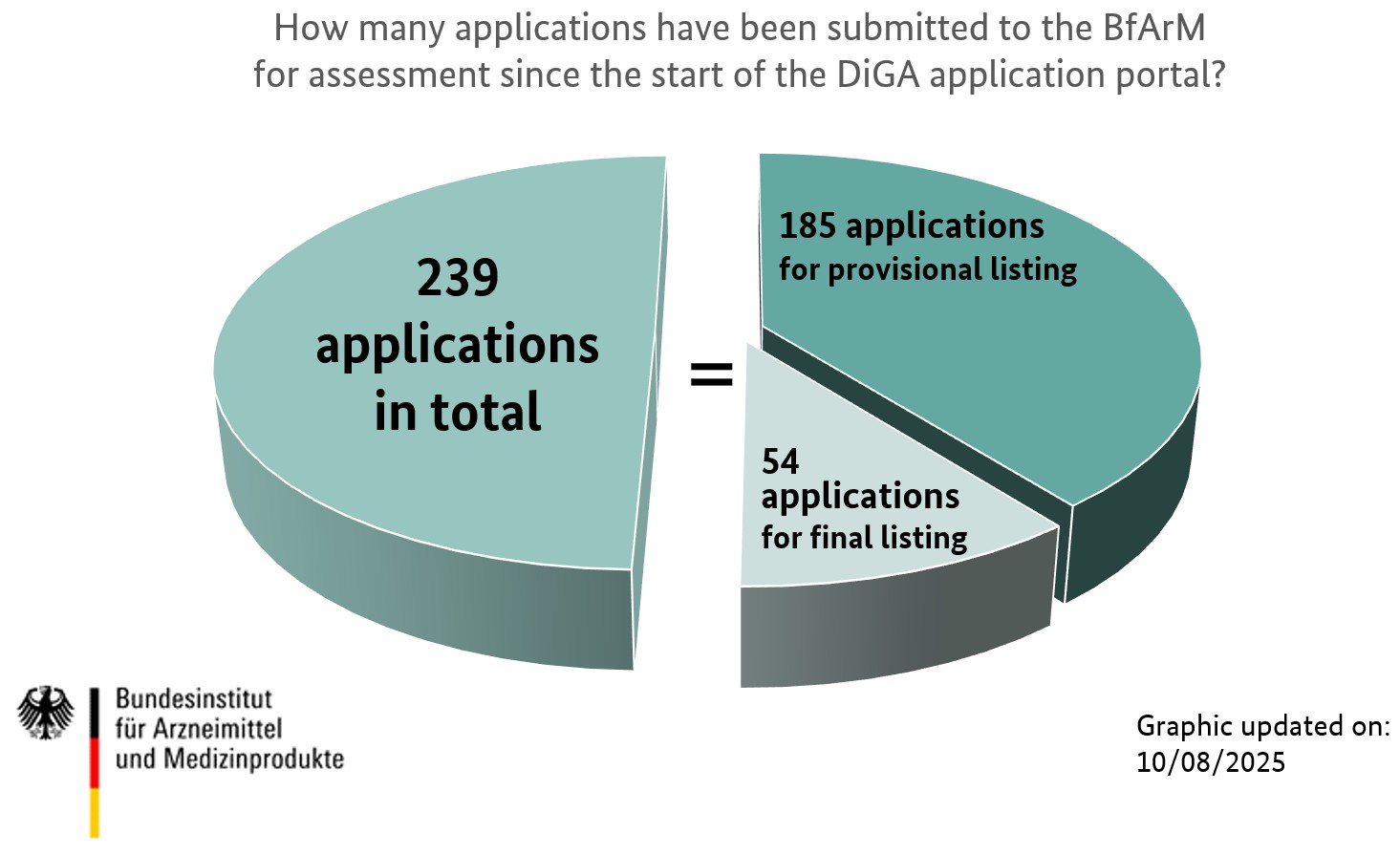
The following figure shows the results of the assessment of applications within the BfArM's DiGA fast-track procedure. "Positive decision" means that the DiGA, for which the manufacturer submitted the application, has been included in the DiGA directory - either for provisional or final listing, depending on the type of application. In contrast, "Negative decision" means that the BfArM's assessment led to the result that the DiGA could not be included in the directory, as it did not fulfil the necessary criteria. "Withdrawn" shows how many applications were retracted by the applicant during the BfArM's assessment process so that no final decision has been taken by the BfArM. "Currently being processed" illustrates the number of applications the BfArM is presently evaluating.