Joint Commission of Experts on the Classification of Substances

Tasks
In January 2013, the Federal Office of Consumer Protection and Food Safety (BVL) and the Federal Institute for Drugs and Medical Devices (BfArM) established an independent Joint Expert Commission - Commission on the Classification of Borderline Substances, which are placed on the market as food or food ingredient. Since then, the 13-member commission has met three times a year, alternating between the BVL and BfArM. On 10th March 2022, the first meeting after the reconstitution for the fourth calling period of the Joint Expert Commission took place as a video conference due to the pandemic.
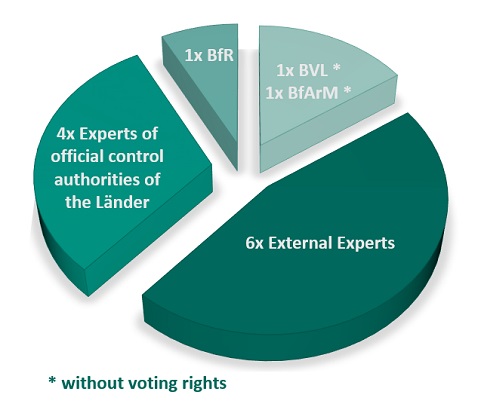
The Joint Expert Commission is composed of six members who are external to the authorities, two members each from the food and drug control authorities of the Länder, as well as one member each from the Federal Institute for Risk Assessment (BfR), the BVL, the BfArM and the respective deputies. In order to underline the independence of the Joint Expert Commission, the two members from BVL and BfArM are not entitled to vote. Scientists with a reputation in research and teaching from the fields of toxicology, pharmacology, medicine/nutritional medicine, food and drug analysis, pharmaceutical biology, nutritional sciences as well as food and drug law were appointed as non-authority members. The experts are appointed for three years at a time. Representatives of the Federal Ministry of Health (BMG), the Federal Ministry of Food and Agriculture (BMEL) and other guests of the BVL and BfArM regularly participate as guests and thus do not have voting rights.
The six non-authority members and six deputy members from outside the authorities were appointed by an appointment committee from a large number of qualified applicants.
Current list of members (German only)
The Joint Expert Commission was established because substances that were previously used primarily or exclusively in medicinal products are increasingly being marketed as foods or food ingredients. However, authorities often have concerns about the safety and marketability of these products as food.
The objective of the Joint Expert Committee, as a recognised independent body with broad expertise, is to develop criteria catalogues, decision trees and opinions on technical issues relating to the classification of substances and their marketability as foods or food ingredients, taking into account scientific findings and in the sense of the legal regulation. In this context, classification includes the demarcation of foods from medicinal products, psychotropic substances and narcotics, as well as the examination of the authorisation requirement under food law, which exists, for example, in the case of novel foods, and the examination of safety based on the BfR's safety assessments.
The recommendations of the Joint Expert Commission are intended to provide the competent federal and local authorities (Landesbehörden) with expert opinions that have an advisory character in the performance of their statutory duties. Thus, the Joint Expert Commission makes an active contribution to the health protection of the population.
Interested parties have the opportunity to comment on drafts and elaborations of the Joint Expert Commission within the framework of a pre-publication. For this purpose, the drafts are made available on the websites of the BVL and the BfArM.
Topics for processing in the Joint Expert Commission can be submitted by the highest local authorities (Landesbehörden) as well as the BMG, BMEL, BfR, BVL and BfArM.
The form for submitting topics can be downloaded here:
Agendas and minutes of results
You can find agendas and minutes of results here (German only)
Office
The office of the Expert Commission is managed jointly and equally by the BVL and the BfArM. It provides technical and organisational support to the rapporteurs, who are appointed from among the experts and prepare the statements. It also takes care of the preparation and follow-up of the meetings.
| BVL (Postal address) | BfArM |
|---|---|
| Bundesamt für Verbraucherschutz und Lebensmittelsicherheit Referat 111 Geschäftsstelle Expertenkommission Gerichtstr. 49 13347 Berlin E-Mail: expertenkommission@bvl.bund.de Please send postal mail only to the BVL | Bundesinstitut für Arzneimittel und Medizinprodukte Validierung Geschäftsstelle Expertenkommission Kurt-Georg-Kiesinger-Allee 3 53175 Bonn E-Mail: expertenkommission@bfarm.de |
Commenting on drafts
The Joint Expert Commission on the Classification of Substances offers interested parties the opportunity to comment on draft opinions and glossary terms before they are published in their final version. Interested parties can submit their comments to the Office for a period of four weeks (optionally extendable by the Office). Critical points will be taken into account in the final editing of the drafts.
At present, no drafts of the Joint Expert Commission on the Classification of Substances are available for comment.
Opinions of the Joint Expert Commission on the Classification of Substances
All opinions in the order of their appearance (German only).
